Transport & Logistics

In recent years, the number of temperature-sensitive biopharmaceuticals and pharmaceutical products has increased immensely.
Combined with the increasing globalization of manufacturing, the transportation of these products is becoming a real logistical
problem. GDP and FDA regulations, such as "EC Guidelines on Good Distribution Practice of Medicinal Products for Human
Use" or "21 CFR Part 210 cGMP in Manufacturing, Processing, Packing, or Holding of Drugs" oblige pharmaceutical suppliers
and manufacturers to keep an eye on the environmental conditions from the first step to the last step of distribution. The ideal
data loggers for this application are the EBI 3x0 data loggers, which combine high measurement accuracy and excellent
functionality with the ability to generate PDF reports at the point of transport without the need for software. The programming
of the loggers can be done either with the freely available software Winlog.basic or via our configuration website www.ebi300.com.
Combined with the increasing globalization of manufacturing, the transportation of these products is becoming a real logistical
problem. GDP and FDA regulations, such as "EC Guidelines on Good Distribution Practice of Medicinal Products for Human
Use" or "21 CFR Part 210 cGMP in Manufacturing, Processing, Packing, or Holding of Drugs" oblige pharmaceutical suppliers
and manufacturers to keep an eye on the environmental conditions from the first step to the last step of distribution. The ideal
data loggers for this application are the EBI 3x0 data loggers, which combine high measurement accuracy and excellent
functionality with the ability to generate PDF reports at the point of transport without the need for software. The programming
of the loggers can be done either with the freely available software Winlog.basic or via our configuration website www.ebi300.com.
EBI 300 TH
Multi-Use PDF Data Logger with external humidity and temperature probe
Relative humidity monitoring in storages and during transports
EBI 300 TE
Multi-Use PDF Data Logger with external temperature probe
fast, flexible core temperature measurements
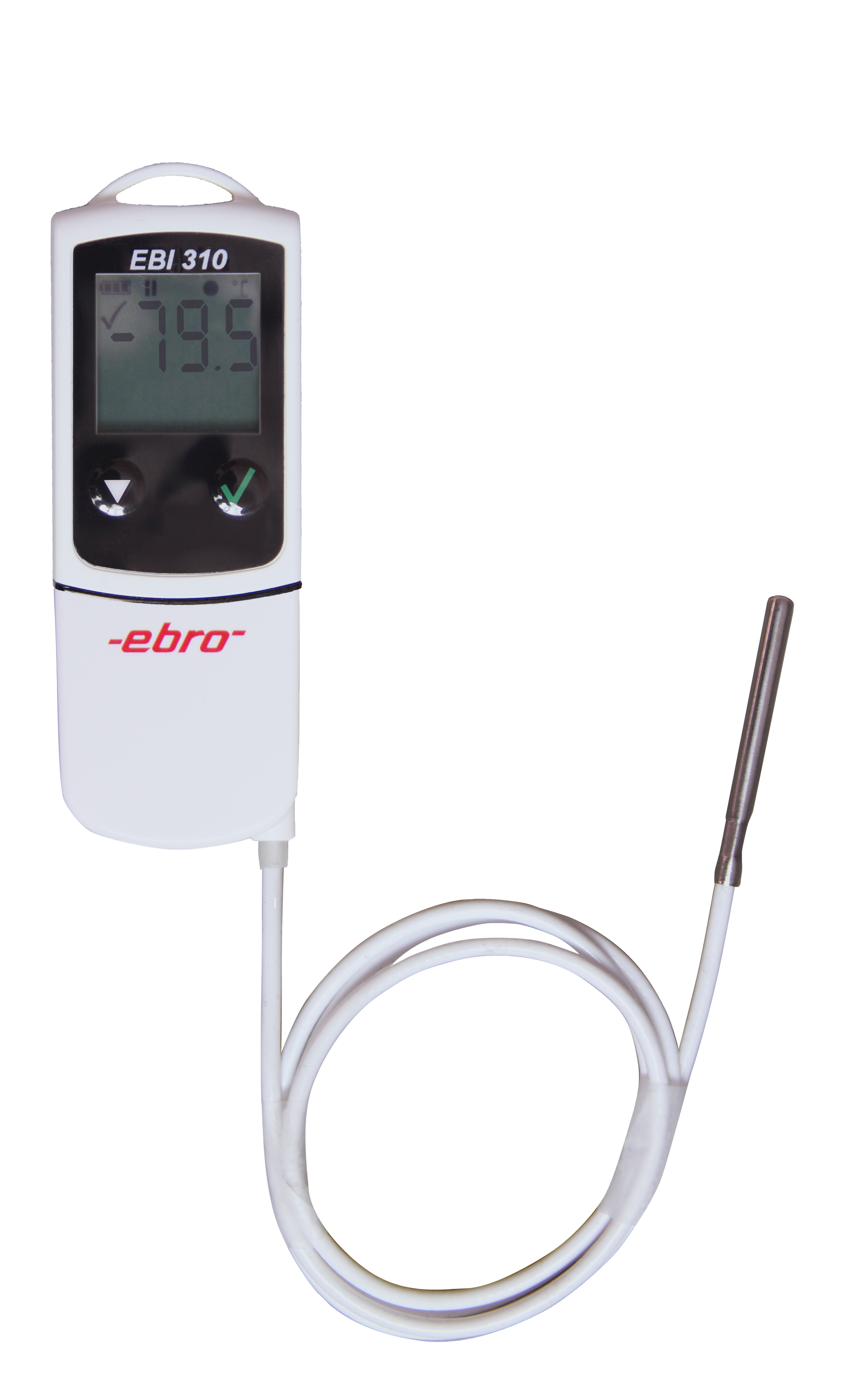
EBI 310 TX
Multi-Use PDF Data Logger
Temperature monitoring in storages and during transport, process monitoring