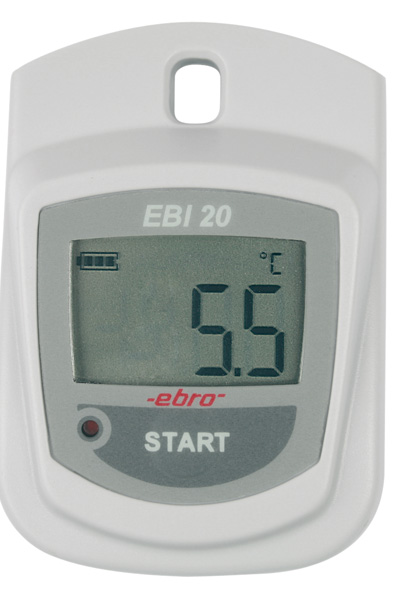
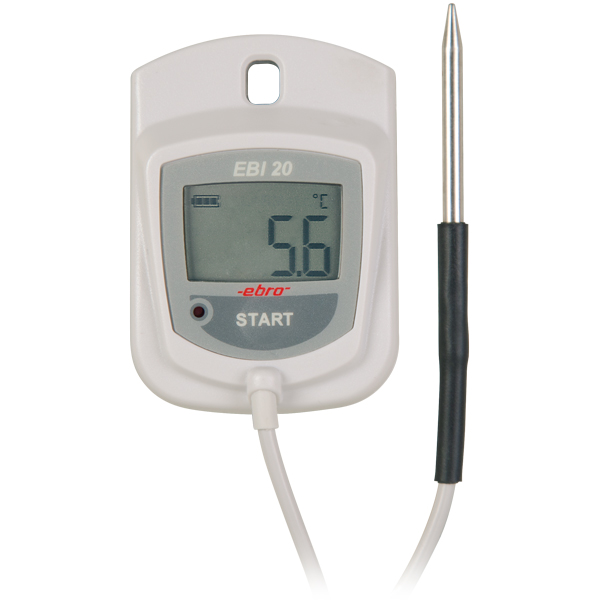
Pharmacie

The requirements of the EU GMP Guide and FDA publications pose major challenges for many manufacturers and suppliers
in the pharmaceutical sector. Our highly accurate and easy-to-use data loggers support you in the implementation of the
guidelines
and specifications regarding transport and logistics, warehousing and the validation and control of sterilization processes.
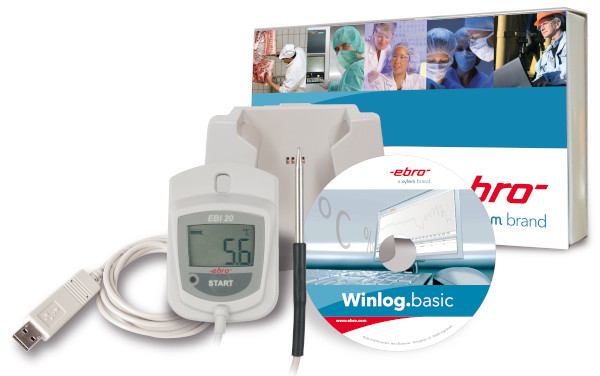
All ebro® software solutions enable user-friendly programming and reading of the data loggers and
are compliant with FDA
requirements regarding data integrity and 21 CFR Part 11.
Transports & Logistique Stockage Validation des processus de stérilisation Solutions logiciels
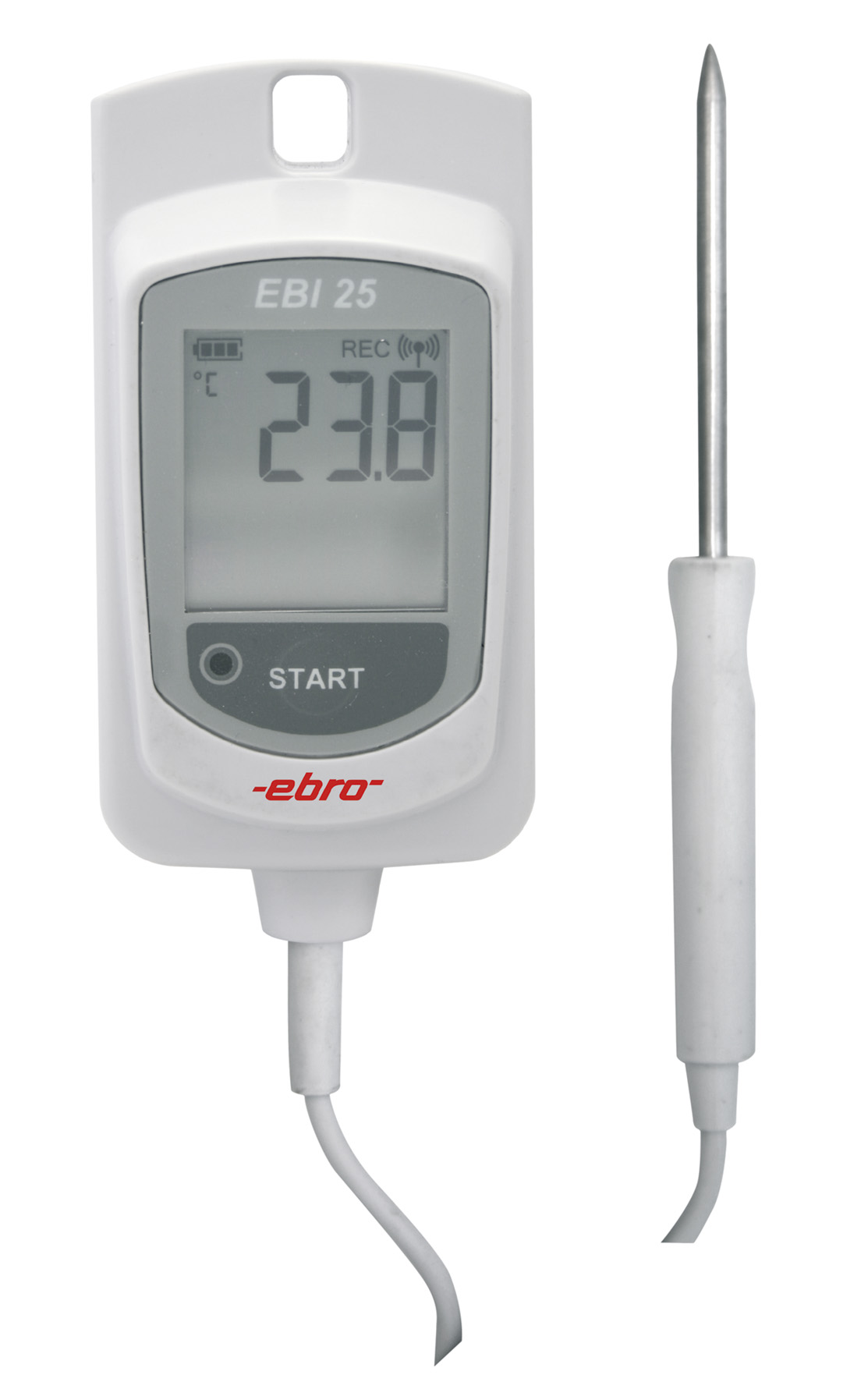
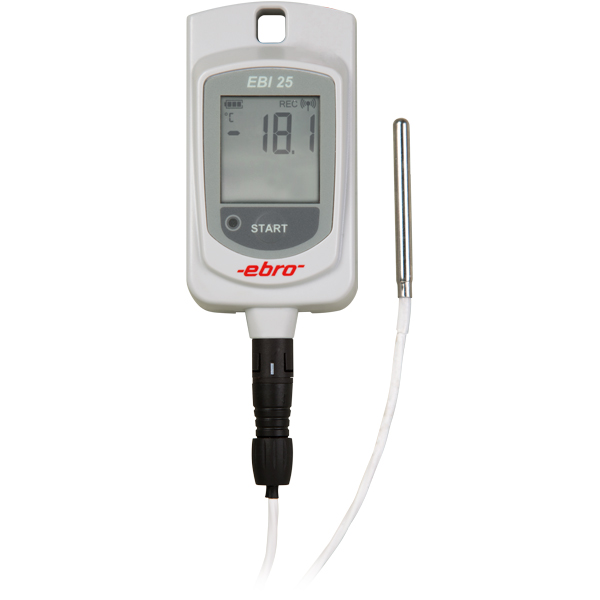
EBI 11-T240
Enregistreur de validation avec temps de réponse rapide
Validation logger with fast response time for use where space is limited
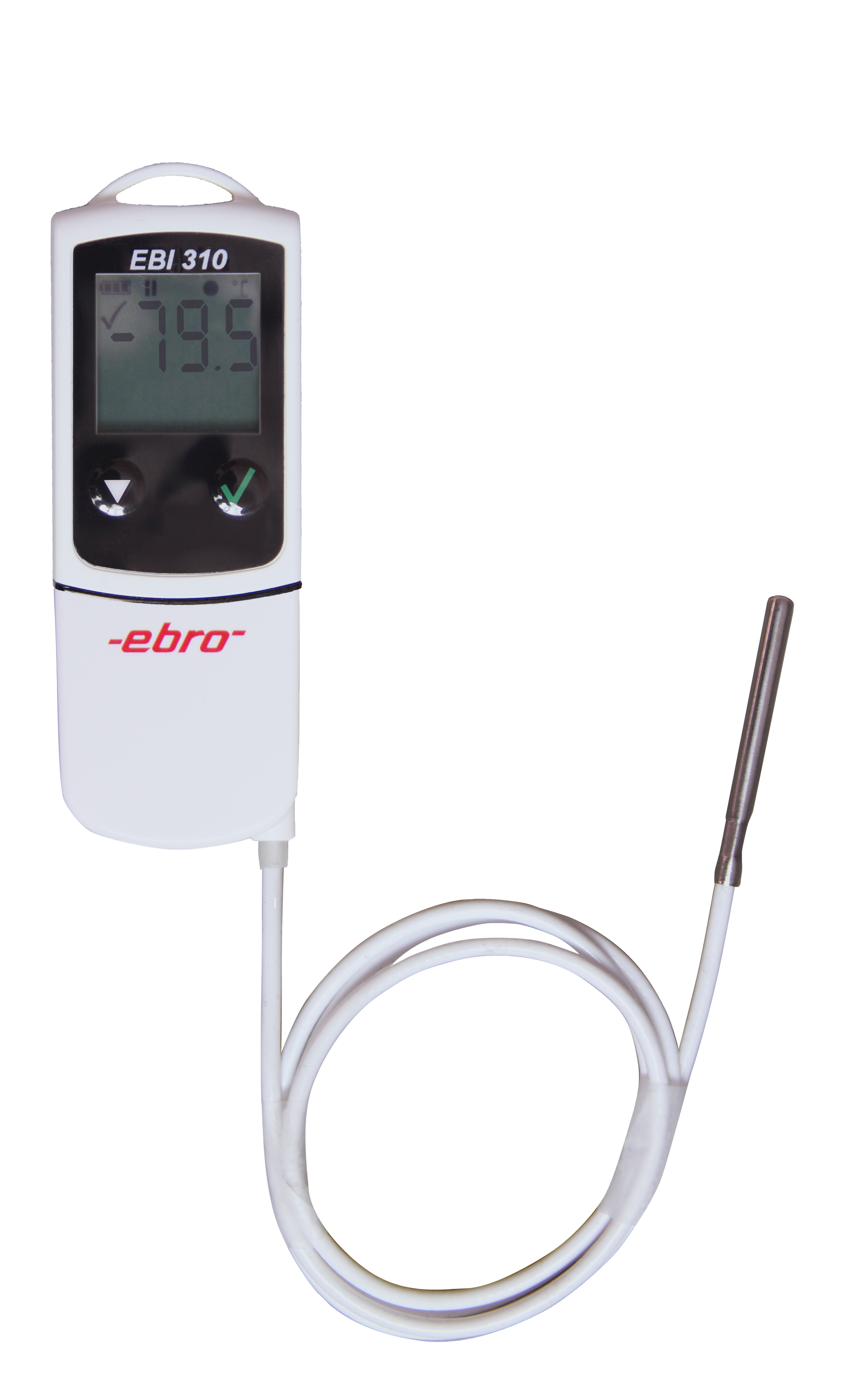
EBI 12-T102
Enregistreur de glace carbonique pour la mesure à -80 °C
Temperature measurement of up to 100 hours in dry ice.