Farmacéutica

The requirements of the EU GMP Guide and FDA publications pose major challenges for many manufacturers and suppliers
in the pharmaceutical sector. Our highly accurate and easy-to-use data loggers support you in the implementation of the
guidelines and specifications regarding transport and logistics, warehousing and the validation and control of sterilization
processes. All ebro® software solutions enable user-friendly programming and reading of the data loggers and are compliant
with FDA requirements regarding data integrity and 21 CFR Part 11.
Transporte & Logística Almacenamiento Validación de los procesos de esterilización Soluciones de software
EBI 310 TX
Registrador de datos - PDF
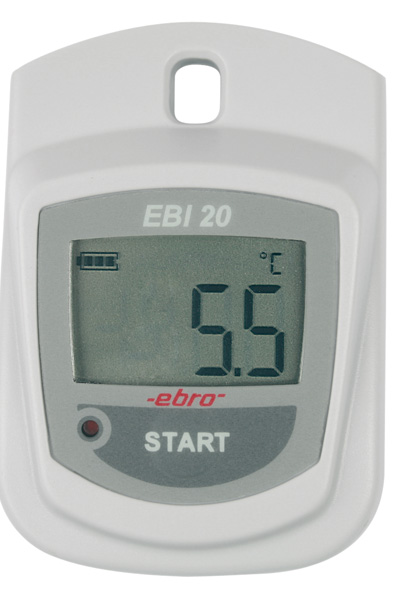
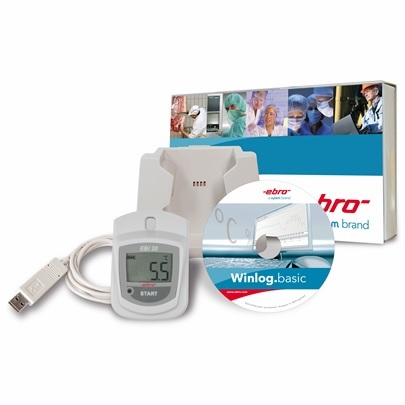
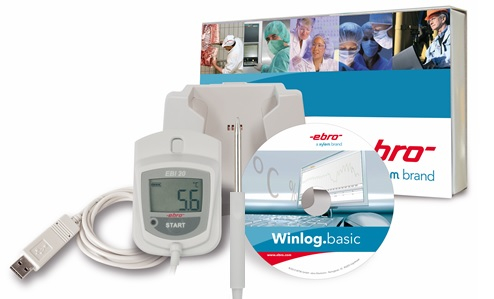
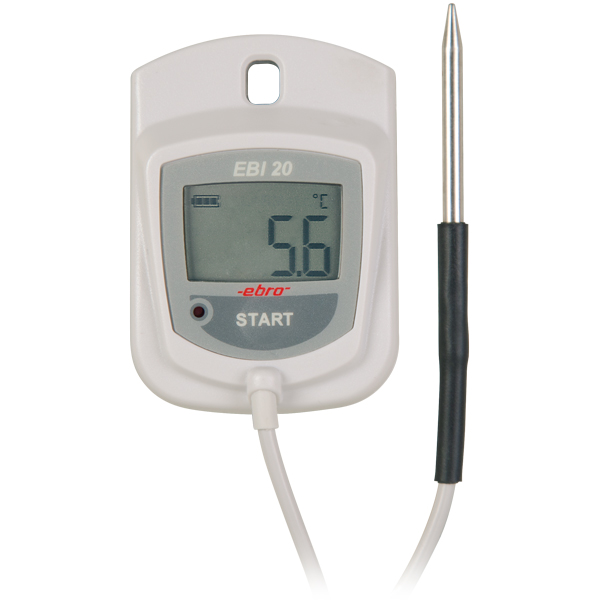
Temperature monitoring in storages and during transport, process monitoring
EBI 11-P100
Mini registrador de datos de presión
Mini validation pressure data logger to messure in steam sterilizers
EBI 12-T222
Registrador de validación versátil
For final inspection after the repair, but also for validation.
EBI 11-T233
Robusto registrador de datos para el control de muestras
Robust temperature data logger for process validation of production processes.
EBI 12-T441
Registrador de validación para mediciones en el esterilizador de vapor
bendable sensor for placement in cavities